find the number of protons se|Protons Neutrons & Electrons of All Elements (List + Images) : Manila How to Find Number of Protons, Neutrons, and Electrons Manhunt is a third-person stealth survival horror video game developed by Rockstar North and published by Rockstar Games, originally released in November 200.
PH0 · Protons Neutrons & Electrons of All Elements (List + Images)
PH1 · Number of Protons, Neutrons, and Electrons in an
PH2 · How to Find the Number of Protons, Neutrons, and Electrons.
PH3 · How to Find the Number of Protons, Neutrons, and Electrons
PH4 · How to Find Number of Protons, Neutrons, and Electrons
PH5 · How To Calculate The Number of Protons, Neutrons,
PH6 · Find the Number of Protons Se
PH7 · Atomic structure calculator (calculating protons, neutrons, electrons)
PH8 · Atom Calculator
PH9 · 4.5: Elements: Defined by Their Number of Protons
PH10 · 2.6: Protons, Neutrons, and Electrons in Atoms
Find all the synonyms and alternative words for odd job at Synonyms.com, the largest free online thesaurus, antonyms, definitions and translations resource on the web.
find the number of protons se*******How To Calculate The Number of Protons, Neutrons, and Electrons
How To Calculate The Number of Protons, Neutrons, and Electrons
How To Calculate The Number of Protons, Neutrons, and ElectronsHow to Find Number of Protons, Neutrons, and Electrons
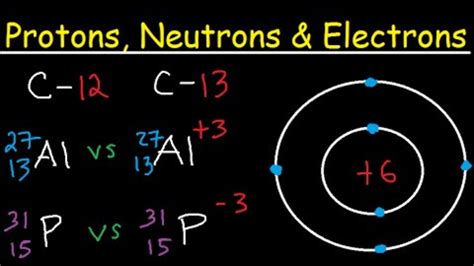
Finding the number of protons, neutrons, and electrons in a given element isn't as hard as it sounds. Oftentimes part of your answer will be right in front of you in the periodic table! Once you know where to look, finding the number of protons, neutrons, . Tingnan ang higit paFind the element Se Se on the periodic table. To find the number of protons in selenium selenium, first locate the element on the periodic table. Next, find the atomic number which is .From the periodic table, we find that it has 29 protons. The mass number (65) is the sum of the number of protons and neutrons. Therefore, we can subtract the number of protons from the atomic number to get the number of neutrons: # n .

The atom calculator is a tool for calculating the atomic number and the mass number based on the number of atom components - protons, neutrons, and electrons (or vice .Protons Neutrons & Electrons of All Elements (List + Images) The number on the bottom left corner is the atomic number, which tells you the number of protons. The number on the upper left corner is the mass number, which is equal to .The easiest way to find the atomic number, is to look on a periodic table, the atomic number is in the upper left corner, or is the largest number on the square. Finding the Number of Protons. . Number of Protons = Atomic Number of the Element. Number of Electrons = Number of Protons. Number of Neutrons = Mass Number - Atomic Number. Find the Number of Protons. Each element is defined by the number .
Atomic structure formulas. Our calculator utilizes these fundamental atomic structure formulas: Atomic Number = Number of Protons. Mass Number = Number of Protons + Number of . Describe the locations, charges, and masses of the three main subatomic particles. Determine the number of protons and electrons in an atom. Write and interpret symbols that .find the number of protons se Protons Neutrons & Electrons of All Elements (List + Images)Counting the number of protons and neutrons tells scientists about the total mass of an atom. \[\text{mass number} \: A = \left( \text{number of protons} \right) + \left( \text{number of .The atomic number or nuclear charge number (symbol Z) of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (n p) or the .find the number of protons seFind the number of protons, electrons, and the charge of N^-? Determine the number of protons and electrons in Ca2+. The charge and identity of an ion with 16 protons, 18 electrons and 17 neutrons would be _____? Determine the number of protons and the number of electrons in Al. Determine the number of protons and the number of electrons in Se. 1.A Se atom has a mass number of 77. Determine the number of neutrons, protons, and electrons in this neutral isotope. number of neutrons: Show transcribed image text. Here’s the best way to solve it.
Since the weight of an atom is (total weight) = (number of protons * 1 amu) + (number of neutrons * 1 amu), we can calculate that 78 = 34 - x (number of neutrons). Subtraction shows that the number of neutrons is 45. So, there are 45 neutrons, 34 protons, and 34 electrons in a neutral Se atom. I hope this helped you!Question: Identify the missing information for each neutral isotope. A Se atom has a mass number of 78. Determine the number of neutrons, protons, and electrons in this neutral isotope. number of neutrons: number of protons: number of electrons: A neutral isotope has a mass number of 65 and 36 neutrons.Atomic Number: Se: Element Symbol: Selenium: Element Name: 78.96: Average Atomic Mass: Step 2. To find the number of protons in , first locate the element on the periodic table. Next, find the atomic number which is located above the element 's symbol. Since 's atomic number is , .2. Your atomic number is the amount of protons within the atom. 3. For neutral atoms, the electron number is the same as the atomic number.otherwise it depends on the charge of the atom.eg: N3- has 3 extra ē. K2± has an absence of ē. You can simply subtract the atomic number from the mass number in order to find the number of neutrons. In this video we’ll use the Periodic table and a few simple rules to find the number of protons and electrons for neutral Nickel (Ni) and the Nickel ions (Ni. Calculate numbers of protons, neutrons, and electrons by using mathematical expressions (1-3): p = 11 (1) n = 23 - 11 = 12 (2) e = 11 - 0 = 11 (3) Alternatively, you can also calculate the atomic number, atomic mass, and charge. Choose your element. Let's assume that it is the sulfide anion. Find the numbers of protons, neutrons, and electrons.If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.Protons and Neutrons in Krypton. Krypton is a chemical element with atomic number 36 which means there are 36 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given .
Because the number of protons remains unchanged when an atom forms an ion, the atomic number of the element must be 13. Knowing this lets us use the periodic table to identify the element as Al (aluminum). . Give the symbol . B Calculate the mass number of each isotope by adding together the numbers of protons and neutrons. C Give the symbol of each isotope with the mass number as the superscript and the number of protons as the . Se Atomic Mass Number (sum of protons and neutrons) = 78. Se Atomic Number (number of protons) = 34. Se Number of neutrons = 78-34 or 44. Now, if this was an uncharged Se atom, we would HAVE to have an equal number of protons (positively charged) and electrons (negatively charged). Boron is a chemical element with atomic number 5 which means there are 5 protons and 5 electrons in the atomic structure.The chemical symbol for Boron is B. Significant concentrations of boron occur on the Earth in compounds known as the borate minerals. There are over 100 different borate minerals, but the most common are: borax, kernite, ulexite etc. .
Write the number of protons, neutrons, and electrons for the following ion. ^(54)_(26)Fe^(2+): _____ _____ _____ Determine the number of protons and the number of electrons in Al. Determine the number of protons and the number of electrons in Se. How do you calculate the total number of protons and electrons in a polyatomic ion? The number of protons in the nucleus is called the atomic number (Z) and is the property that defines an atom’s elemental identity. The sum of the numbers of protons and neutrons in the nucleus is called the mass number and, expressed in amu, is approximately equal to the mass of the atom. An atom is neutral when it contains equal numbers of .Find the atomic mass and mass number of an atom with 6 protons and 8 neutrons. Identify the number of protons, neutrons, and electrons in the following neutral isotope: _{20}^{48}\textrm{Ca} An atom has a symbol of Mo and 53 neutrons. Determine its atomic number, number of protons and electrons, mass number, and atomic mass.
Watch Trixie Lalaine Vidjakol free porn on AsianPinay.com, the best asian and pinoy scandal porn site. . You like this video? You will also like. Buti Nalang Nandiyan si Hipag na Handang Magpakantot Pag Kinakailangan. . Sex after class. 17 00:30. Urbandub Chronicles 17. 85 10:25. Show more related videos. Leave a Reply Cancel reply.
find the number of protons se|Protons Neutrons & Electrons of All Elements (List + Images)